
CRISPR gene editing brings hope to people with inherited blindness
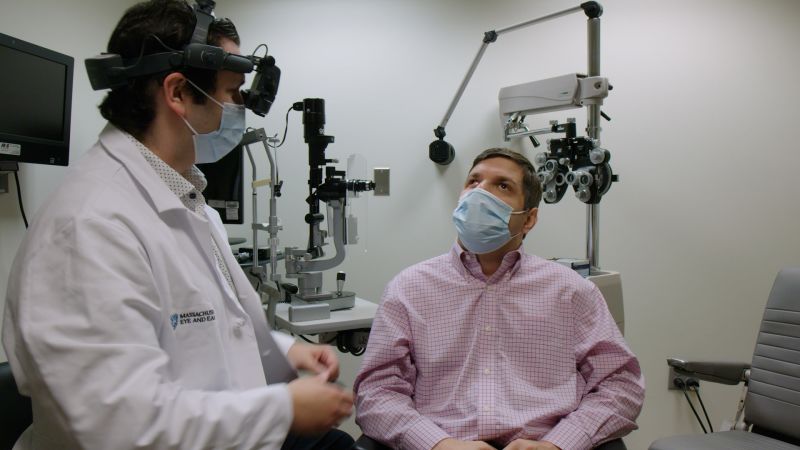
A recent clinical trial, called BRILLIANCE, has shown promising results in restoring vision for individuals with a rare form of inherited blindness known as Leber Congenital Amaurosis (LCA). The study was led by Eric Pierce, MD, Ph.D., of Mass Eye and Ear and involved 14 participants who received the gene therapy treatment EDIT-101.
The trial demonstrated that CRISPR-Cas9 gene editing is a safe and effective method for correcting the CEP290 gene mutation, which is responsible for LCA. The results showed that 11 out of the 14 participants experienced improved vision without serious adverse side effects.
The study marks an important milestone in the field of gene therapy as it is the first to use this approach to treat children born with a form of blindness. Participants reported being thrilled to be able to see food on their plates and other objects that were previously unseen.
Blindness in LCA is caused by a gene mutation that prevents the CEP290 protein from functioning properly, which is critical for sight. The participants received a single dose of CRISPR gene therapy called EDIT-101, which cuts out the mutation in CEP290 and replaces it with a healthy strand of DNA, restoring normal function of the protein and allowing the retina to detect light.
The success of this clinical trial is a significant step forward in treating inherited blindness using gene editing techniques. The findings were published in The New England Journal of Medicine on May 6, 2024.
Two other studies reported similar results around the same time, with Children's Hospital of Philadelphia physicians restoring eyesight for two children and Olivia Cook, a college student from Missouri State University, experiencing improvements in her vision after receiving the experimental gene-editing treatment. These studies further validate the potential of CRISPR gene editing as a viable solution for treating inherited blindness.
The future of CRISPR gene editing
More than two hundred people have been treated with experimental CRISPR technologies, but only one CRISPR treatment has been approved for clinical use so far - Casgevy, a treatment for sickle-cell disease. Scientists are entering a new phase in genome editing technologies where they can help and cure patients with a variety of diseases not just treat them safely. Ongoing clinical trials are testing other CRISPR therapies for HIV/AIDS, diabetes, cancers, cardiovascular diseases, and antibiotic resistance.


/cloudfront-us-east-1.images.arcpublishing.com/pmn/VTZPPU6T5RFIVPUXZER4KTBNRA.jpg)