In recent years, there has been a significant shift in the use of GLP-1 receptor agonists (GLP-1 RAs) from treating type 2 diabetes to obesity. According to several studies, this trend began around 2020 and has continued since then. In this article, we will explore the reasons behind this shift and its implications.
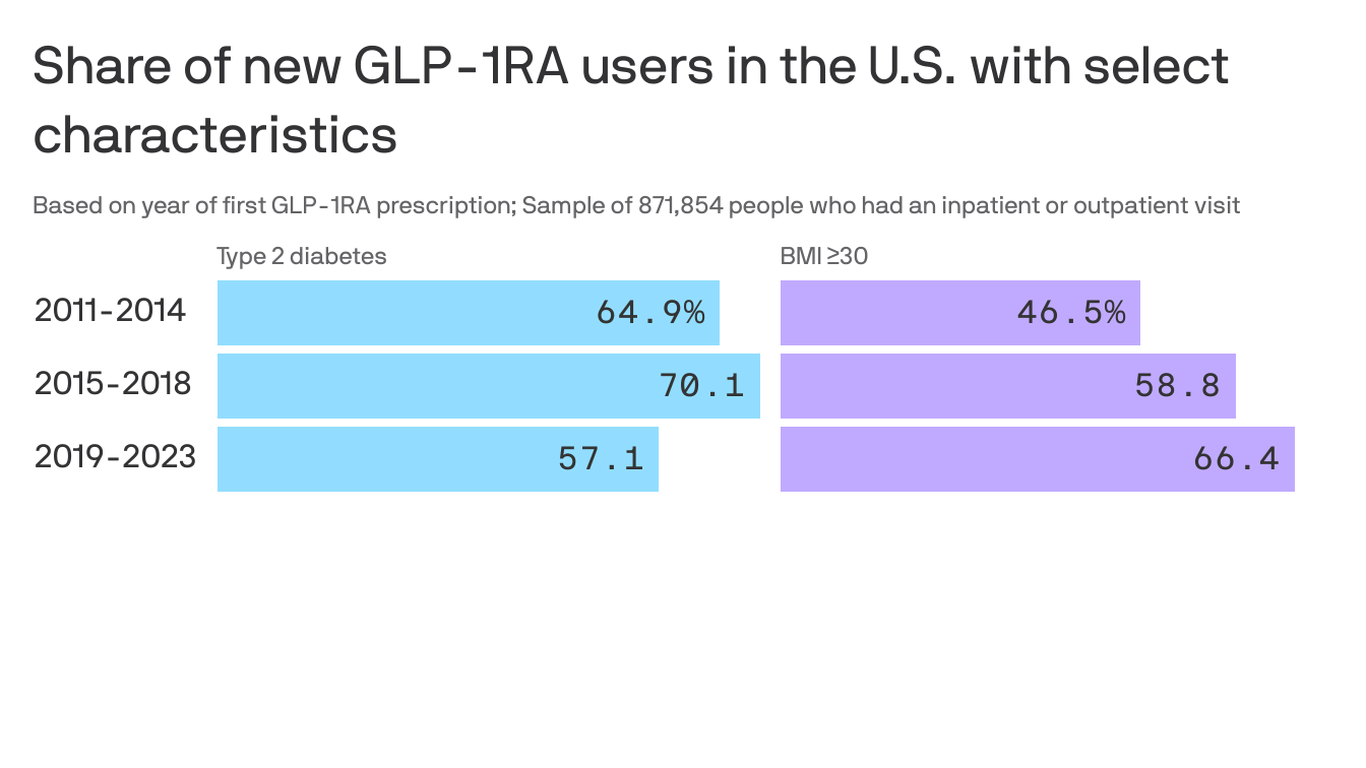
Firstly, let's examine the data. Between 2011 and 2023, approximately one million new GLP-1 RA users were identified in the United States (Yee Hui Yeo et al., Annals of Internal Medicine). During this period, there was a decrease in the proportion of new GLP-1 RA users with type 2 diabetes and an increase in those without it but with a body mass index (BMI) indicative of obesity or overweight and an obesity-related comorbid condition. For instance, between 2019 and 2023, the proportion of new GLP-1 RA users with type 2 diabetes dropped from approximately 57% to around 43%, while those without it but with a BMI of equal to or greater than 30 kg/m² rose from nearly 47% to over 66% (Rezaie et al., Annals of Internal Medicine).
Furthermore, semaglutide, the active ingredient in Novo Nordisk's diabetes drug Ozempic and their anti-obesity drug Wegovy, was the most prescribed GLP-1 RA in 2023. It accounted for over 88% of new prescriptions (Yee Hui Yeo et al., Annals of Internal Medicine).
The reasons behind this shift are multifaceted. One factor is the FDA's approval of GLP-1 RAs for weight management indications, such as obesity and weight loss in combination with diet and exercise. For instance, semaglutide was approved for chronic weight management in 2021 (Food and Drug Administration).
Another factor is the high prevalence of obesity in the United States. According to the Centers for Disease Control and Prevention, approximately 42.4% of adults in the United States had obesity in 2017-2018 (Centers for Disease Control and Prevention).
However, this shift towards using GLP-1 RAs for obesity has implications. For instance, it may contribute to drug shortages due to the high demand for these medications. Additionally, it may exacerbate racial and ethnic disparities in access to these drugs since they are disproportionately prescribed to non-Hispanic white females with a BMI of equal to or greater than 30 kg/m² (Yee Hui Yeo et al., Annals of Internal Medicine).
In conclusion, the shift towards using GLP-1 RAs for obesity instead of type 2 diabetes has been significant in recent years. This trend is driven by FDA approvals and the high prevalence of obesity in the United States. However, it also raises concerns about potential drug shortages and disparities in access to these medications.